Cell culture requires sterile conditions to avoid having unwanted microorganisms growing with the cells of interest. This means that the environment where you keep and manipulate your cells has to be perfectly sealed (when closed) or completely microorganism-free. The way this is achieved is by manipulating cells under what is called a laminar flow hood, which is a hood that purifies and circulates air so that the flasks containing cells can be open and exposed to air without the risk of microorganisms entering. Those flasks are then closed and kept inside incubators that are regularly disinfected so all risks of contamination are minimized. Precautions to take when in the room include:
- ensuring the door is properly closed, as the pressure inside the room is kept higher so that air from outside cannot come in
- manipulating flasks with care
- minimizing the exposure of skin to the air
- avoiding talking in order not to spread germs (we all have them, by the way).
Operating in the laminar flow hood
The laminar flow hood is a sterile space where you can manipulate cells safely. However, in order to maintain sterility, you should follow a few rules. When using the laminar flow hood, always spray your gloves with ethanol and clean the hood after each use with Virkon (lab bleach) and ethanol. Always spray with ethanol items that you’re introducing such as flasks, bottles of medium, pipettes (even if they’re sterile, the plastic outside has still been in contact with ambient air).
Operator asepsis
We all have thousands of microorganisms in our body: from our skin, to our mouth and hair. It is very important to keep those microorganisms from entering the hood. PPE helps isolate those areas and minimize their contact with air: your lab coat and gloves are great for this. But many times other cell culture-specific protection is needed: plastic sleeves, to avoid having areas between the lab coat sleeve and gloves unprotected; a face mask, to avoid breathing air out into the hood (this is especially important if you have a cold); scrub hats, especially for ladies with long hair (alternatively keep your hair tied up); shoe covers, in order to avoid introducing contamination from outdoors (some people prefer to keep a special pair of shoes at the entrance of the lab).
Flasks and bottles
- Spend as little time as possible with flasks and bottles open inside the hood.
- Never exchange caps between bottles
- Never leave caps screw down, leave them with the flat top surface on the hood surface and away from the work area.
- Never share bottles of medium or reagents between different cultures (cell lines). Split them in two new bottles and open them separately for each cell line.
Cell culture flasks
Always open them when inside the hood and when you’re going to use their contents. Never tilt them in a way that can cause the liquid to come into contact with the cap. Do not use micropipettes to take a sample if they do not reach the surface of the liquid. Depending on the flask type, the cap is closed by fully screwing it to the end (if it has a filter in the cap) or you need to screw it until you hear a ‘click’ sound. This is so the flask has the same oxygen and CO2 concentration as the incubator and your cells can ‘breathe’.
General materials
Common sterile materials to use in a laminar hood include micropipette filter tips, micropipettes, lab tweezers, centrifuge tubes, a pipette gun etc… All of these should previously have been sterilized (e.g. tweezers go in the autoclave) or come sterile (micropipette tips). Some labs use the same for everyone and just leave the open materials in the hood for the next person to use, other labs have every user bring and store their own. Ask a colleague how it’s done in your lab.
Using vacuum to remove cell culture waste
When changing medium with adherent cells, or sometimes when removing supernatants after spinning cells, it is very common to use a vacuum pump to remove the old medium before adding the new one. There are a few precautions when using it:
- Use a new Pasteur pipette every time you change flask.
- Make sure the Pasteur pipette is properly inserted in the vacuum pump tube (or risk a spillage).
- Always remember to empty the waste container when it starts to get full (and regularly anyway) to avoid contamination.
Using a centrifuge
Centrifuging (or spinning) cells is a method that is used very frequently to split cells from the liquid they are in. The principle is really simple: it’s a washing machine put sideways. The cell suspension is placed in a centrifuge tube, which is a special tube that has a screw cap so the liquid cannot come out, and the centrifuge tube is placed in a special holder that can slide in the radial direction (this is for larger centrifuges; for the smaller ones, it’s just a fixed diagonal holder). Once the sample is in and the lid is closed, centrifugation starts, which means the samples rotate around the centrifuge axis at a very high speed. This pulls cells, which were distributed evenly around the liquid, towards the bottom of the centrifuge tube and separates them physically from the liquid. This is very useful because the supernatant (liquid above the cells) can then be removed with a pipette and cells can be resuspended in a different liquid (new medium for example). It can also be used to wash cells from a reagent or to prepare them for analysis.
Centrifuges have several settings: the time you want to centrifuge for, the ramp for speed increase and speed decrease, and the speed itself (some even have a heating or cooling option). Speed can be specified in two ways: RCF or RPM. RCF, or relative centrifugal force, refers to the speed relative to your machine diameter. It is a measure of the g-force applied to the sample (same as when you’re in a turn with a car and you feel it pulling you towards a side). RPM, on the other side, stands for revolutions per minute, so it accounts for the speed around the center, or the time it takes (in minutes) for a tube holder to rotate once around the center. They are equivalent, and the formula to convert RPM into RCF is the following:
As you can see, you will only need RPM and the radius R (in mm) to do so. The radius is measured directly on the machine: it’s the distance between the axis of the centrifuge and the bottom of a tube holder put sideways (aligned horizontally). When following protocols, either of RCF or RPM will be given. Centrifuges generally accept input in both units; if so, just select the required RPM, or the required RCF and the radius.
Below is a calculator to convert between both.
Below are two examples of centrifuge tube arrangements inside the centrifuge sliders. Note that tubes should be symmetrical with respect to the center point of the centrifuge (not vertically or horizontally). Top: arranging an even number of tubes with the same volume in different ways. Bottom: arranging an odd number of tubes with sample and adding an additional tube with water containing exactly the same volume to balance the weight, two possible arrangements.
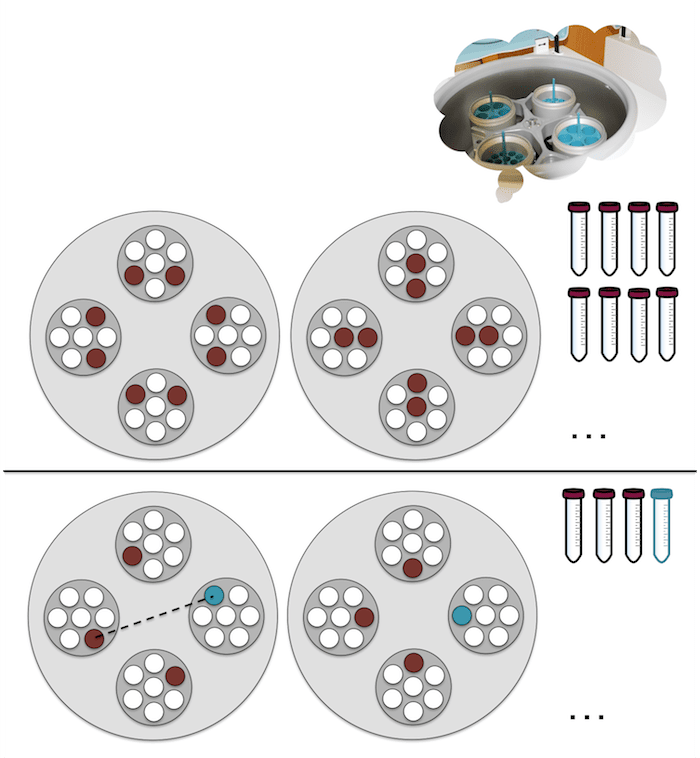
Some precautions to take when using a centrifuge are:
- always close the tube caps
- balance the weight of the tubes so that the same amounts are placed in diametrically opposite holders (on each side of the centrifuge, see the figure above)
- never use it if the holders are broken
- if it starts vibrating, press the stop button and check everything is in order before using it again
- never place delicate items around it as it might break them and injure someone if it vibrates out of control
